Intestinal Health : Macrophages lead the conversation between the microbiota and the colon
Researchers from Institut Curie, Inserm, and CNRS discover a new mechanism by which unique macrophages control the quality of the fluids absorbed by the intestinal epithelium. They protect colonic cells from the toxic substances produced by microbiota.
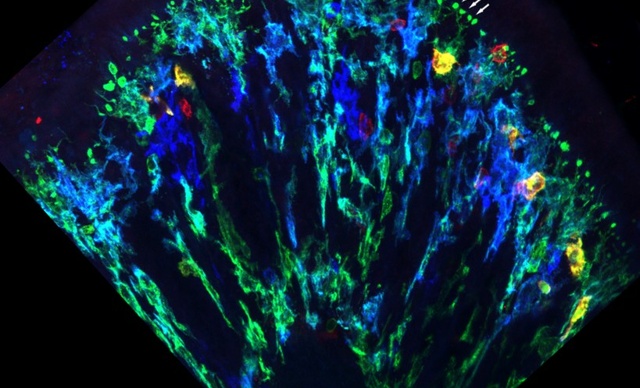
Coupe de côlon dans laquelle on observe les macrophages subépithéliaux et leurs BLP en vert.
Maintaining the integrity of the intestinal barrier is critical, and its disruption can result in multiple pathological situations. Researchers from Institut Curie, Inserm, and CNRS discover a new mechanism by which unique macrophages control the quality of the fluids absorbed by the intestinal epithelium. They protect colonic cells from the toxic substances produced by microbiota. Their research, which was published in Cell, reveals part of the hitherto unknown dialog between the immune system, microbiota, and the intestinal epithelium and marks a breakthrough in our understanding of the mechanisms by which homeostasis and intestinal balance are maintained.
The intestinal tract comprises different regions, each with its own distinct anatomy and physiological role. The distal colon, which includes the descending colon and the sigmoid colon, is particularly important for processes linked to digestion and the absorption of fluids such as water and nutrients. Microbiota in this region house a tremendous number of microorganisms, including an abundance of fungi that can produce substances that are toxic to the cells. What are the mechanisms by which the colonic mucosa protects itself from these toxic fungal substances while still absorbing the nutrients it needs? How does the organism manage to maintain the integrity of the intestinal barrier in this area of the colon?
The “Spatio-temporal dynamics of immune cells” research group at Institut Curie and Inserm[1], directed by Ana-Maria Lennon, and the Cell migration and invasion research group at Institut Curie and CNRS[2], directed by Danijela Matic Vignjevic, collaborated to research the tight regulation of fluid absorption by the colonic mucosa, the mechanisms of which were hitherto largely unknown.
The researchers[3] studied a unique sub-population of macrophages (immune system cells). In response to signals sent by fungi in colonic microbiota, these macrophages form “balloon-like” protrusions (BLP). The researchers showed that when absorbed fluids contain toxic metabolic fungi, these BLP insert themselves at the base of the colon epithelium and control the quality of the fluids absorbed by the epithelial cells. The macrophages can then “inform” the epithelial cells about the presence of toxic substances so that they can stop the absorption of fluids and protect themselves from poisoning and cellular death. The researchers showed – in vivo – that in the absence of either the “balloon-like” protrusions or the macrophages, the epithelial cells continue to absorb toxic fluids, which leads to both their death and loss of intestinal barrier integrity.
“We had no idea that we would discover this unexpected and vital mechanism by which macrophages in the distal colon facilitate the recognition of toxic fluids by colonic cells and stop their absorption, thereby maintaining epithelial integrity and local homeostasis,” states Ana-Maria Lennon, director of research at Inserm and head of the Institut Curie research team.
What is the nature of the signals exchanged by fungi and macrophages? What are the means by which macrophages send signals to alert the colonic epithelium? How do the epithelial cells receive this information? These questions remain unanswered, but studying them will help us not only apprehend a suite of physiological processes and also understand certain pathologies. Indeed, changes to the integrity of the intestinal barrier are responsible for several diseases such as ulcerative colitis and Crohn’s disease.
Danijela Matic Vignjevic, director of research at Inserm and head of the Institut Curie/CNRS research team, explains, “We still have a number of questions to answer before we will be able to decrypt the signals being exchanged between fungi and microbiota, macrophages and epithelial cells... our objective is now to understand, on a cellular and molecular level, how these signals are transmitted so that we can apprehend or even block the pathological processes.”
|
|
See on YouTube |
[1] This group, directed by Inserm’s Ana-Maria Lennon, is part of the Immunity and Cancer unit (Inserm/Institut Curie/Université de Paris, PSL University).
[2] This other group, led by Inserm’s Danijela Matic Vignjevic, is part of the (CNRS/Institut Curie/Sorbonne University, PSL University) Cell Biology and Cancer unit.
[3] In collaboration with scientists from the Immunology Center at Marseille-Luminy (CNRS/Inserm/AMU).
|
Référence : Macrophages maintain epithelium integrity by limiting fungal product absorption. Aleksandra S. Chikina, Francesca Nadalin, Mathieu Maurin, Mabel San-Roman, Thibault Thomas-Bonafos, Xin V. L, Sonia Lameiras, Sylvain Baulande, Sandrine Henri, Bernard Malissen, Livia Lacerda Mariano, Jorge Barbazan, J. Magarian Blander, Iliyan D. Iliev, Danijela Matic Vignjevic and Ana-Maria Lennon-Duménil. |


