Team
Genetics of Tumor Suppression
Thematic areas of research:
Image

To top
Team
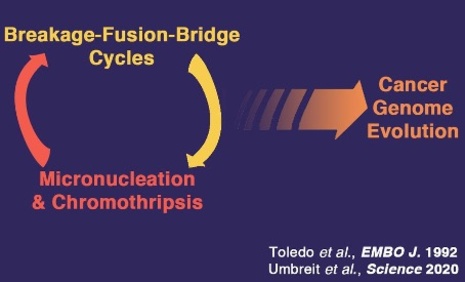
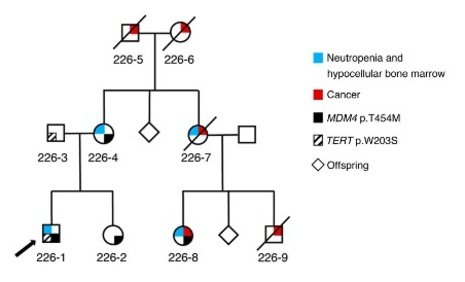
FRANCK TOLEDO
Genetics of Tumor Suppression
The p53 pathway is impaired in most, if not all, tumors. In half of human cancers the p53 gene is mutated, and in the other half the p53 protein can be inactivated by overexpression of one of its specific inhibitors, MDM2 or MDM4.
Members

Image

Key publications
All publications
-
p53 in the Molecular Circuitry of Bone Marrow Failure SyndromesInternational Journal of Molecular Sciences
-
A systematic approach identifies p53-DREAM pathway target genes associated with blood or brain abnormalitiesDisease Models & Mechanisms
-
-
p53 downregulates the Fanconi anaemia DNA repair pathwayNature Communications
-