- Home >
- Institut Curie News >
- ESMO 2025 : Finding Less Toxic Treatments for Women with Hormone-Dependent Breast Cancer
Preliminary results from the RIBOLARIS clinical trial, promoted by Solti (Spain) and coordinated by Unicancer (France), show that in more than 50% of patients, chemotherapy — usually prescribed after surgery to reduce the risk of recurrence — could be replaced with a less toxic treatment, significantly improving their quality of life. These promising results will be presented in an oral session by Prof. Paul Cottu, medical oncologist at Institut Curie, on October 17, 2025, during the annual ESMO Congress to be held in Berlin.
Breast cancer is the most common cancer among women, with more than 60,000 new cases diagnosed each year in France1. Of those, 70% to 80% are hormone-dependent breast cancers.
The RIBOLARIS clinical trial, promoted by Solti in Spain and coordinated by Unicancer in France, is a multicenter international study co-led by Professor Paul Cottu, medical oncologist at Institut Curie, and Professor Aleix Prat, medical oncologist at the Hospital Clínic Barcelona2.
The study aims to evaluate the effectiveness of chemotherapy-free treatment for patients with hormone-dependent breast cancer who would normally be prescribed chemotherapy after surgery to reduce the risk of recurrence.
In France, 35 centers are participating, including Institut Curie, which has the largest cohort with 68 patients.
Since April 2022, 1,100 patients have been enrolled in the study, and the preliminary results from the first 686 patients are being presented at the ESMO Congress.
These patients, all with stage 2 or 3 hormone-dependent breast cancers, received preoperative treatment combining targeted therapy (ribociclib, a CDK4/6 inhibitor) and hormone therapy (letrozole) for six months before undergoing surgery.
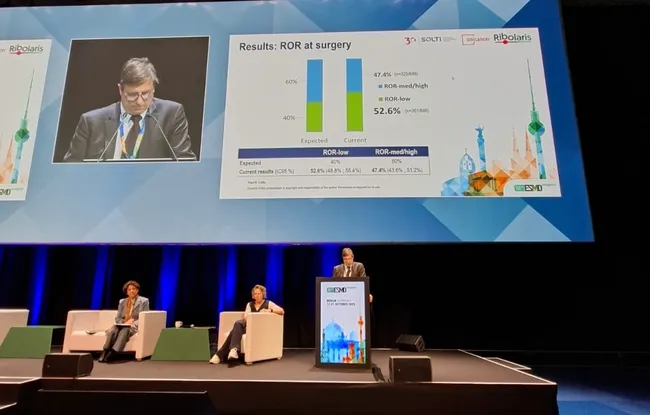
The physicians then used the genomic risk-of-recurrence (ROR) score to tailor subsequent treatment. Based on that score, the patients either continued with the preoperative treatment alone (targeted therapy + hormone therapy), or received chemotherapy in addition to the preoperative treatment.
The goal was to use the molecular profile of each patient’s tumor, as determined after the preoperative treatment with letrozole and ribociclib, as a guide for the adjuvant treatment.
“What is innovative about this trial is that a new neoadjuvant approach could become the preferred strategy for hormone-dependent breast cancers,” explains Prof. Aleix Prat, medical oncologist at the Hospital Clínic Barcelona. “RIBOLARIS is the first trial to use a highly validated genomic tool such as the CE-marked ROR tool to guide decision-making and choose post-surgery therapy.”
“We had anticipated that chemotherapy could be avoided in 40% of patients, but the results have exceeded our expectations: so far, more than 52% of patients have been able to avoid chemotherapy,” says Prof. Paul Cottu, medical oncologist at Institut Curie. “We have just recruited the last patients, who will undergo surgery between now and March 2026. We will then move on to a five-year monitoring phase, after which we hope to define clear criteria for adjusting and personalizing treatments and avoiding chemotherapy whenever possible while reducing the risk of recurrence. In the future, we hope to change treatment practices for 30–40% of women with hormone-dependent breast cancer and thereby improve their quality of life.”
[1] French National Cancer Institute (INCa), 2023.
[2] Director of the Comprehensive Cancer Center in Barcelona, Spain; Head of the Translational Genomics and Targeted Therapies Group, IDIBAPS; Professor at the University of Barcelona.