- Home >
- Institut Curie News >
- Institut Curie launches the first study to assess the clinical utility of circulating tumor DNA in the evaluation of chemotherapy response
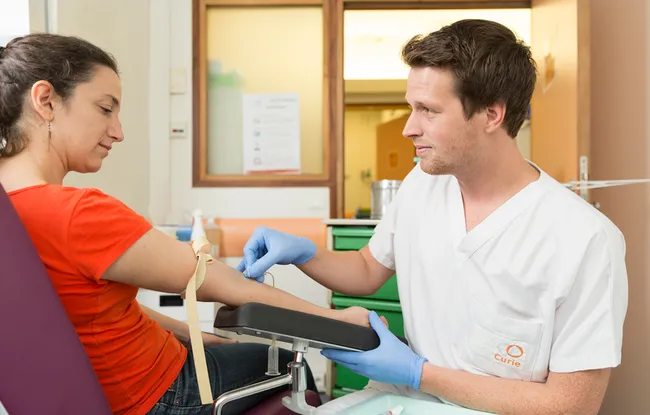
The MONDRIAN clinical trial[1] assesses the clinical utility of monitoring early chemotherapy response using circulating tumor DNA (ctDNA) for patients with triple-negative metastatic breast cancer.
This trial is a global first and the culminations of years of Institut Curie research, says Professor François-Clément Bidard, medical oncologist and principal investigator of the trial. We are very proud to be launching it with Institut Curie patients, thanks to funding from public donations, community and public funds, and the support of a Swedish biotech company[2].
Today, evaluating the performance of a new chemotherapy relies mainly on X-ray examinations and can take months. Monitoring quantitative changes in tumor DNA molecules circulating in the blood could allow us to quickly know if a cancer is responding to chemotherapy or not.
This groundbreaking trial could radically change the way metastatic disease is managed: reducing the time required to assess treatment effectiveness avoids irradiation and gives clinicians an advantage by enabling them to offer new chemotherapies before cancer progresses.
Monitoring ctDNA will also allow doctors to rule out chemotherapies that are not adequately effective on a given cancer, thereby reducing both prolonged exposure and side effects.
The trial, which has just launched, will include 214 patients and last three-and-a-half years.
[1]MONDRIAN stands for My OwN cIRculating biomarker tumour DNA
[2] The MONDRIAN clinical trial was funded by the following: fundraising at the 2nd Institut Curie Golf Open (2019), SIRIC funds, 2019 Ruban Rose Avenir prize awarded to François-Clément Bidard, SAGA Society, 2019 Henry and Mary-Jane Mitjavile Award awarded to Prof. François-Clément Bidard by the National Academy of Medicine.