- Home >
- Institut Curie News >
- Cancer under pressure
With my initial background in physics, I would never have imagined that we would find that mechanics had such an important role in the physiopathology of cancer, potentially useful to medicine
,explains Emmanuel Farge, Inserm research director and manager of the Mechanics and Genetics of Embryonic and Tumor Development team (CNRS UMR168/Sorbonne University) at Institut Curie
But along with his colleagues, he uncovered the surprising role of mechanical pressure in development of colon cancer in mice. This discovery opened up new avenues of treatment for many different types of cancer in humans.
Double the number of stem cells
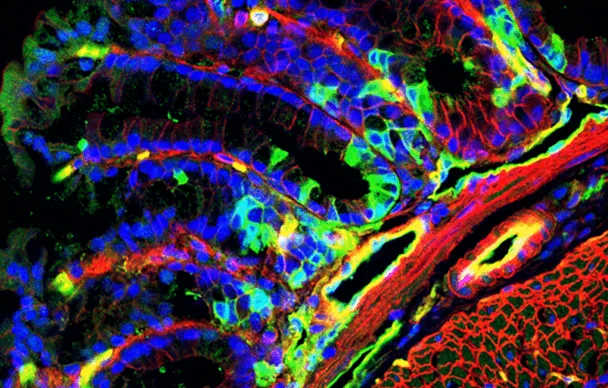
Emmanuel Farge, in collaboration with Maria Elena Fernández-Sánchez and her teams, discovered that the mechanical activation of a biochemical pathway known as ß-catenin by natural contractions of the colon is needed to maintain the physiological quantity of stem cells in the colon, and that, on the other hand, the over-activation of this pathway by the permanent growth pressure of a tumor leads to a pathological doubling of the number of proliferating stem cells. They also discovered an increase in cancerous stem cell markers, a sign that hyperproliferation seems to give the cells the ability to invade the tissues and resist treatment.
They therefore continued their exploration of the biological mechanisms involved and confirmed in vivo that a protein known as Ret kinase plays a role upstream of the ß-catenin pathway in mice. And then the team of Didier Meseure, medical manager of the Pathex experimental pathology platform, at the Diagnostic and Theranostic Medicine division at Institut Curie’s Hospital Group, confirmed that this same biochemical pathway was over-activated in human colon cancer cells and that this over-expression was also present in nine other malignant solid tumors. These observations could produce targeted therapies for many types of cancer, particularly cancers of the ovary, lung and pancreas, which has a poor prognosis. With this in mind, researchers successfully tested in vivo the resorption by half of spontaneous intestinal tumors in mice by treating with molecules known to block Ret kinase. As with many inhibitors, these molecules are also known for their high level of toxicity. The thing that now motivates Emmanuel Farge and his colleagues is that:
we have to find a way to act on Ret in a less toxic or non-toxic way for the entire body
,he explains.
Mechanical pressure and cancer
About twenty years ago, Emmanuel Farge suspected an effect of mechanical pressure on cell differentiation, meaning the acquisition of functions specific to each type of cell, and was able to check it with his team during embryo development. He also drew a possible parallel with the formation of cancerous tumors, and the confirmation of this parallel led to a publication in the journal Nature. In previous work, his team had also shown a biochemical pathway involved in these “mechanical sensitivity” (or mechanical transduction) phenomena, a pathway known as Wnt/ß-catenin.
References
Communications Biology
https://doi.org/10.1038/s42003-022-03079-4
Ret kinase-mediated mechanical induction of colon stem cells by tumor growth pressure stimulates
cancer progression in vivo
Thanh Huong Nguyen Ho-Bouldoires 1,8, Kévin Sollier 1,8, Laura Zamfirov 1,2,8, Florence Broders-Bondon 1,8, Démosthène Mitrossilis 1,3, Sebastian Bermeo 1, Coralie L. Guerin 4, Anna Chipont 4, Gabriel Champenois 5, Renaud Leclère 5, Nicolas André5, Laurent Ranno 6, Aude Michel 7, Christine Ménager 7, Didier Meseure 5, Charlie Demené 2, Mickael Tanter 2, Maria Elena Fernández-Sánchez 1,9✉ & Emmanuel Farge 1,9✉
1 Institut Curie, Université PSL, Sorbonne Université, CNRS UMR 168, Laboratoire de Physico-Chimie Curie, Mechanics and Genetics of Embryonic and Tumoral Development team, INSERM, F-75005 Paris, France.
2 Physics for Medicine Paris, ESPCI ParisTech, PSL Research University, Inserm U1273, F-75005 Paris, France. 3 Biomedical Research Foundation of the Academy of Athens, 4 Soranou Ephessiou St., 115 27 Athens, Greece.
4 Cytometry Platform, Institut Curie, Paris, France.
5 Platform of Investigative Pathology, Institut Curie, 75248 Paris, France.
6 NEEL Institut, CNRS, Grenoble Alpes University, F-38042 Grenoble, France.
7 Sorbonne Université, Laboratoire PHENIX Physico-chimie des Electrolytes et Nanosystèmes Interfaciaux, CNRS UMR 8234, F-75005 Paris, France.
8 These authors contributed equally: Thanh Huong Nguyen Ho-Bouldoires, Kévin Sollier, Laura Zamfirov, Florence Broders-Bondon.
9 These authors jointly supervised this work: Maria Elena, Fernández-Sánchez, Emmanuel Farge. ✉email: maria-elena.fernandez-sanchez@curie.fr; emmanuel.farge@curie.fr