- Home >
- Institut Curie News >
- Characterization of two molecules modulating cardiac contraction: one step further in precision medicine
FHC is a public health problem affecting around one in 500 people. Clinically, these diseases are characterized by abnormal contractions of the heart, leading to heart failure over time.
“These abnormal contractions are due to mutations in the contractile muscle fibers called myosins, which act as the pump's motor. We know that there are several hundred of these mutations today, with different molecular effects, but similar symptoms,” explains Dr. Julien Robert-Paganin, a researcher in the team of Anne Houdusse, CNRS research director.
Today's treatments rely on invasive surgeries, including heart transplants in the later stages of the disease, with serious consequences for patients' quality of life.
“One question arises from these data: is it possible to develop drugs capable of rectifying the contraction force of the heart by directly modulating myosin activity?” continues Anne Houdusse.
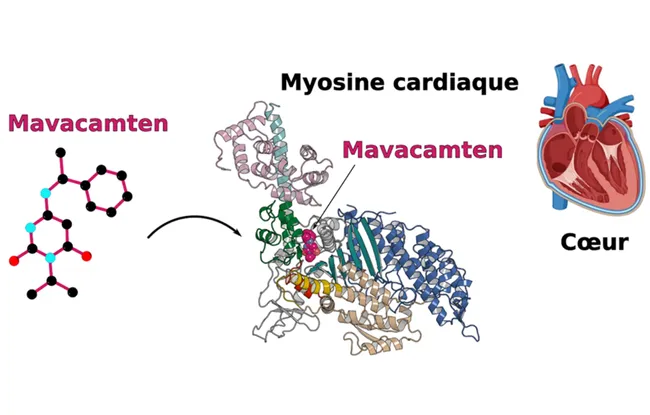
Two molecules have these effects: Omecamtiv mecarbil, which activates myosin contraction, and Mavacamten, which inhibits it. To understand how these molecules mediate their opposing effects, Dr. Julien Robert-Paganin and his colleagues used X-ray crystallography to identify the binding site on myosin. The study reveals that these two molecules, despite having opposite effects on myosin contraction, bind to exactly the same spot on the muscle fiber. Advanced molecular dynamics studies, developed with Dr. Daniel Auguin (Université d'Orléans), show how these two drugs mediate their opposite effects. Although their target is the same, the molecules do not have the same mobility and stability in the pocket, interacting differently with the motor domain of myosin, which explains their opposite actions.
In addition to treating the symptoms, these molecules have been shown to slow disease progression. Thickening of the heart wall, the cause of long-term complications, is due to the abnormal force of contraction of the heart muscles. Studies have shown that by rectifying this force, the walls are less damaged, suggesting that taking this type of medication in the early stages of the disease can reduce the risk of developing heart failure.
For the remainder of this project, the team is continuing to combine structural studies with analysis of molecular dynamics to characterize other therapeutic molecules.
“Our team is also comparing the action of other molecules of therapeutic interest. To develop precision medicine, we now need to test the efficacy of these drugs for various heart diseases,” says Julien Robert-Paganin.
Anne Houdusse's team aims to better understand how muscle contraction is controlled, to diversify molecules of interest capable of modulating the force produced.
“This study is a key step in our understanding of modulators of molecular motor function. Now that Mavacamten has just been made available to patients, our research provides essential knowledge to inform doctors about its mode of action and better advise them on future revolutionary treatments for heart disease now in clinical phase 3,” concludes Anne Houdusse.
|
Reference: Daniel Auguin, Julien Robert-Paganin, Stéphane Réty, Carlos Kikuti, Amandine David, Gabriele Theumer, Arndt W. Schmidt, Hans-Joachim Knölker, Anne Houdusse Omecamtiv mecarbil and Mavacamten target the same myosin pocket despite antagonistic effects in heart contraction. Nature Communications (xx xx 2024) – DOI: https://doi.org/10.1101/2023.11.15.567213
Link to read the publication : https://pubmed.ncbi.nlm.nih.gov/38014327/ |