- Home >
- Institut Curie News >
- New results from Institut Curie shed light on environment-dependent collective cell migration.
These results, obtained by Dr. Mathilde Lacroix in Dr. Pascal Silberzan's team in the Physics of Cells and Cancer laboratory (UMR168 Institut Curie / CNRS / Sorbonne Université), were published in Nature Physics on May 27th, and provide a better understanding of the mechanisms involved in triggering and organizing collective cell migration on a fibrillar surface.
Collective cell migration, or the organized and directed movement of a group of cells, is an essential phenomenon in organogenesis or tumor maturation. "In this process, cells move together in a veritable obstacle course. These obstacles can be other tissues, but also microscopic structures in the extracellular matrix. Our team, at the interface between physics and biology, is interested in precisely these issues of scale: how do the microscopic properties of the cells' environment influence their behavior on a group scale?" explains Pascal Silberzan.
Cellular currents
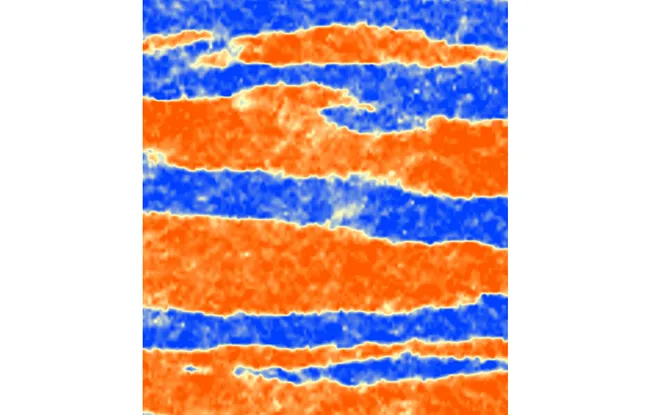
Using microfabrication techniques, the cell culture surface was textured with microni-sized grooves that mimic the presence of microscopic extracellular matrix fibers. Although each cell covers several of these patterns, the researchers observed that cell movements are structured collectively along these grooves in bidirectional currents several dozen cells wide. In the absence of these grooves, cells exhibit disordered movements, confirming that they are at the origin of the cell currents observed.
Unraveling the mechanisms of cell migration
Although these cells do not adhere to each other, they coordinate like individuals in a compact crowd, leading to the emergence of collective behavior through a mechanism involving their polarity, i.e. the asymmetry existing between front and back, facilitated by the surface anisotropy (cell displacements being easier along the rails than perpendicular to them). In addition to the experiments, theoretical modelling and numerical simulations carried out in collaboration with Carlès Blanch-Mercader and Jacques Prost, researchers in the Physics of Cells and Cancer laboratory (UMR168 Institut Curie / CNRS / Sorbonne Université), and Bart Smeets (Catholic University of Leuven, Belgium), have enabled the results obtained on the bench to be interpreted more quantitatively.
"We have thus shown that microscopic surface anisotropy can control the collective organization of cell displacements and induce very broad band-structured currents. In our experimental model, anisotropy is entirely controlled by microfabrication. However, in vivo, cells constantly modify their substrate by depositing and modifying molecules. It will therefore be interesting to include this component in future experiments to describe in vivo behavior as closely as possible, while retaining our physical approach. Not only do our experiments provide a better understanding of the mechanisms involved in morphogenesis, for example, but, in the longer term, this knowledge could find applications in tissue engineering" concludes Pascal Silberzan.
Reference: Mathilde Lacroix, Bart Smeets, Carlès Blanch-Mercader, Samuel Bell, Caroline Giuglaris, Hsiang-Ying Chen, Jacques Prost et Pascal Silberzan
Emergence of bidirectional cell laning from collective contact guidance. Nature Physics (27 May 2024) doi: 10.1038/s41567-024-02510-3
Description of the photo : When plated on microgrooved substrates, bronchial cells migrate collectively. Their displacements organize in wide antiparallel lanes. The orange and blue colors code for the opposite directions of the flows within these lanes. Therefore, subcellular cues have the potential to shape large scale collective cell flows. Image is 1.5 mm wide.