- Home >
- Institut Curie News >
- A new imaging approach for tracking cell fate
There are a wide variety of neurons in the brain, orchestrating many functions, from muscle control to the elaboration of complex thoughts. Derived from stem cells, neurons must undergo differentiation and migration before they can perform their functions in neural networks.
“The rise of single cell techniques has made it possible to demonstrate the huge diversity of neurons and stem cells in the brain. Thanks to these techniques, we have data at a given moment in time, but they don't provide us with information on the mechanisms underlying the development of this diversity: we don't know which stem cells produced which neurons, what the differentiation stages were, or even where these divisions took place,” says Dr. Alexandre Baffet, Head of the Cell Biology of Mammalian Neurogenesis team (CNRS UMR 144/PSL Université) at Institut Curie.
Mapping cell fates
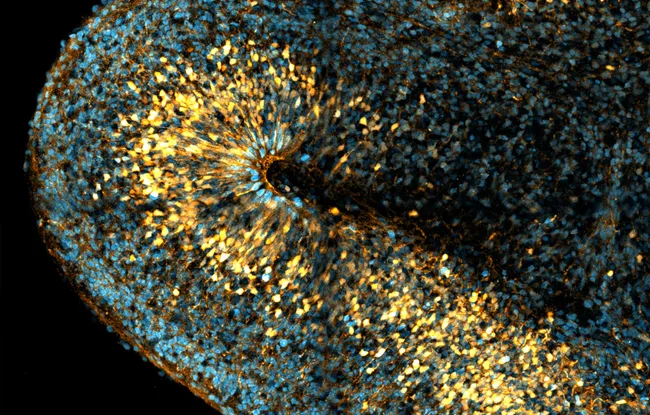
To solve these problems, researchers in Alexandre Baffet's team have developed an imaging method to follow the destinies of a particular group of stem cells, known as radial glia cells or bRGs.
Researchers filmed the cells for long periods, up to a few days, to visualize and characterize bRG divisions. After analyzing this data, they were able to map cell divisions in organoids (1). This mapping is consistent with that established in tissues. “A promising first result, which supports the use of organoids as a model in studies of human nervous system development”, explains Alexandre Baffet.
Cell fates, evolution and pathology
The neocortex, the seat of higher cognitive functions such as decision-making, is particularly well-developed in the human brain. “Today, this recent evolutionary expansion is attributed to the presence of large numbers of bRGs during embryonic development. In our study, we observed a significant amplification of bRGs, supporting the current hypothesis: these cells are indeed involved in the expansion of the neocortex, and it is they that make, in large part, our brain human” continues Alexandre Baffet.
The remainder of this project will focus on the role of microenvironmental factors influencing cell destinies, to further unravel the drivers of this neuronal diversity. “And we also have collaborations in the pipeline: this technique can be used to study any cell line, from any organ. For example, we have a project with Geneviève Almouzni as part of the PEPR Cell-ID program, to study the aberrant cell fates involved in the development of pediatric tumors,” concludes Alexandre Baffet.
(1) human tissue cells in culture forming structures similar to those found in the brain
Reference: Laure Coquand, Clarisse Brunet Avalos, Anne-Sophie Macé, Sarah Farcy, Amandine Di Cicco, Marusa Lampic, Ryszard Wimmer, Betina Bessières, Tania Attie-Bitach, Vincent Fraisier, Pierre Sens, Fabien Guimiot, Jean-Baptiste Brault & Alexandre Baffet.
A cell fate decision map reveals abundant direct neurogenesis bypassing intermediate progenitors in the human developing neocortex. Nature Cell Biology (March 28, 2024) - DOI: https://doi.org/10.1038/s41556-024-01393-z