- Home >
- Institut Curie News >
- From C. elegans to human life: the rhythm in the genes
During development cells multiply by successive division to ensure growth and reproduction. This process is above all a question of genetics: the right genes must activate at the right time, for a precise duration and in the right order. The cells that lose rhythm and escape control can cause tumors to occur.
We want to understand what keeps the genes on alert and why this regulation is lacking in cancer. We chose to observe what happened in stem cells in the C. elegans nematode worm, and we discovered a genetic clock that shows similarities with the human circadian rhythm
explains Dr. Wolfgang Keil, head of the Quantitative Developmental Biology team (CNRS UMR168/Sorbonne University).
The many benefits of the C. elegans nematode worm
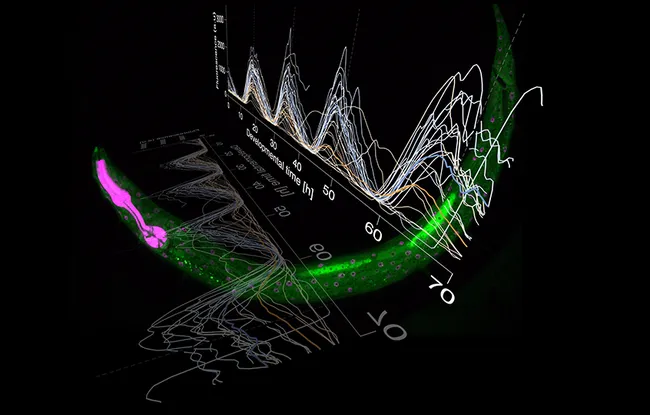
The Caenorhabditis elegans worm is in fact an excellent model for this study, and has been used for decades to identify genetic function in vertebrates in physiological or pathological processes. It is transparent at all stages of its life, which enables researchers to observe each cell division in real time and gene expression under a microscope. In addition, it reproduces very rapidly such that one can track its entire development, from fertilization to adulthood, in just three days. Furthermore, its growth follows a regular pace which is already well characterized. It is therefore easy to identify any malfunctions.
The C. elegans worm has no circadian rhythm. But it has genes that are very similar to genes that control day/night cycles in mammals. Until now very little was known about the role of these genes in the worm.
We imagined that the original genetic circadian rhythm transformed during the course of evolution to regulate the pace of development of this simple organism.
The genes programming development of C. elegans
The study conducted by the team of Dr. Wolfgang Keil in collaboration with the team of Dr. Christopher M. Hammell from Cold Spring Harbor Laboratory showed that two proteins, called NHR-85 and NHR-23, which are similar to the mammalian circadian rhythm genes Rev-Erb and ROR, regulate the development of the C. elegans nematode.
Because the worm is very active, we designed an imaging technique to gently immobilize it for long enough to observe its gene expression in real time over many hours. In this way we discovered that NHR-85 and NHR-23 acted together to trigger a “pulse” of gene expression of the lin-4 microRNA, to punctuate each step in the development of the C. elegans’ stem cells
he explained.
The two teams also discovered how another protein, LIN-42, is involved in this process. LIN-42 interrupts the expression of the lin-4 microRNA at each stage of development by blocking the action of the NHR-85 protein. Intriguingly, LIN-42 is very similar to another mammalian circadian rhythm gene called Period. “When we produce a mutation in the genes of this “clock”, miRNA gene expression no longer happens at the right time and at the right pace,” concludes Dr. Wolfgang Keil. “Now we’d like to understand whether the clock that we have discovered in C. elegans also regulates expression of other genes. Other studies have shown that the development of the worms is faster in hot conditions and slower at low temperatures. How can the rhythms be different while the pacing of the steps remains the same? This is what we now want to discover.”
This study was financed with the Institut Curie JPI Starting Package and with the IC3i PhD grant program financed by Institut Curie and the European Union.