- Home >
- Institut Curie News >
- New insights on how material properties help cancer cells move and spread
Cell migration is not only central to these processes but also plays a role in diseases like cancer, where it can contribute to the spread of tumors. In a new study published in Developmental Cell, Dr. Matthieu Piel (CNRS UMR144/Sorbonne University/Institut Curie) and Dr. Raphaël Voituriez (CNRS UMR8237/Sorbonne University/Laboratoire de Physique Théorique de la Matière Condensée) a universal framework for understanding “amoeboid” cell migration—a type of movement that occurs without the cell attaching to surfaces and is especially relevant for immune and cancer cells.
Animal cells, although often stationary in their tissue, possess an intrinsic ability to move. This movement is essential for processes such as embryonic development, wound healing and immune responses, but it can also be hijacked in cancer, allowing cells to spread throughout the body. The migration of so-called amoeboid* cells is a complex process that relies on cellular “muscles” (the actomyosin cytoskeleton) and other factors.
The movement of Amiboid cells
The study led by Dr. Matthieu Piel (CNRS UMR144/Sorbonne University/Institut Curie) and Dr. Raphaël Voituriez (CNRS UMR8237/Sorbonne University/Laboratoire de Physique Théorique de la Matière Condensée) reveals that amoeboid cells, instead of containing a homogeneous skeleton, divide their internal structure into three distinct regions, each with specific properties that help the cell to move.
“Until now, most research assumed that amoeboid cells contained a single, uniform network of actin and myosin,” explains Dr Juan Manuel García-Arcos, 1st author of the study, ex-doctoral fellow of Matthieu Piel and post-doctoral fellow at the University of Geneva. “Instead, we discovered that these cells create distinct zones: a flexible or soft front that deforms to squeeze into small spaces, a rigid center to maintain the shape, and a back that serves as a 'muscle' to push the cell forward. This organization enables the cells to generate force and move efficiently.”
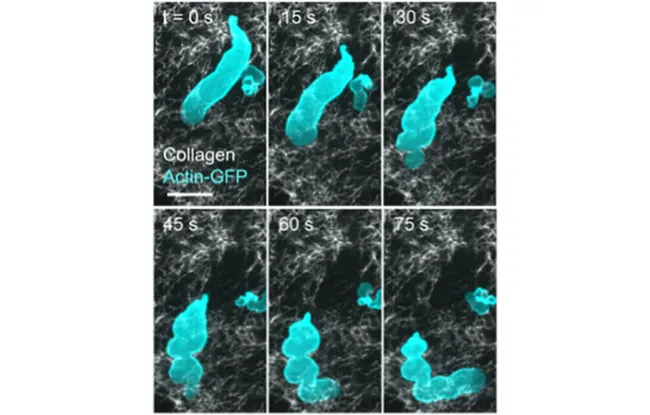
To explore amoeboid migration, the researchers created a simplified cellular model in which confined human cells formed “blebs” or protrusions that eventually break off into small, mobile fragments. These fragments mimic real cell behavior, with organized actin and myosin structures supporting independent movement. This model enabled the team to observe how these networks of cellular “muscles” interact to propel movement.
Fragmentation, the driving force behind cell propagation
This study goes a step further, shedding new light on a process known as cell fragmentation - when cells divide into small, mobile pieces. Observed here for the first time in a controlled environment, fragmentation appears to be a natural consequence of confined amoeboid movement. This discovery echoes recent microscopy studies in living tumors, where scientists have observed cancer cells fragmenting in real time, with pieces breaking off and moving independently through the tissue.
The implications are profound. The ability of cells to fragment and produce mobile fragments capable of moving around the body could explain how cancer cells spread more easily, or how immune cells mobilize to fight infection. Each fragment retains an organized internal structure, enabling it to move autonomously. This discovery broadens our understanding of cell migration and opens up new avenues for studying cancer metastasis and immune cell behavior. This advance suggests that targeting the mechanisms behind cell fragmentation could lead to new therapies aimed at limiting cancer spread or enhancing immune responses, offering a new perspective for controlling these processes in health and disease.
*type of cell that can change shape: immune cells such as macrophages, for example, are amoeboid cells, which enable them to move around in tissues, or perform functions such as phagocytosis.
Publication: https://doi.org/10.1016/j.devcel.2024.06.023
Video: https://youtu.be/bgwz0PbiQFQ
Reference : Juan Manuel García-Arcos, Johannes Ziegler, Silvia Grigolon, Loïc Reymond, Gaurav Shajepal, Cédric J Cattin, Alexis Lomakin, Daniel J Müller, Verena Ruprecht, Stefan Wieser, Raphael Voituriez, Matthieu Piel
Rigidity percolation and active advection synergize in the actomyosion cortex to drive amoeboid cell motility. Developmental Cell, 2024 July 20 doi : 10.1016/j.devcel.2024.06.023
This sequence of images taken with a fluorescence microscope shows the dynamics of actin (in cyan) and collagen fibers of the extracellular matrix (in white). A small cell fragment, marked by actin, detaches from the main structure and moves independently through the collagen network. The scale bar represents 10 micrometers.
Research News
Discover all our news
Celebration
The Immunity and Cancer research unit (U932) celebrates its twentieth anniversary
12/12/2025
Artificial Intelligence
08/12/2025