- Home >
- News
Institut Curie News
Discover the latest news from Institut Curie.
Search
Filter
Filter
Publication date
Fields
Search
227 result(s)
- Celebration
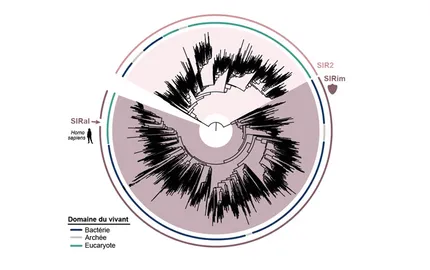
The Immunity and Cancer research unit (U932) celebrates its twentieth anniversary
12/12/2025
- Artificial Intelligence
SHAIPED: Institut Curie integrates a consortium of experts to create a "European space for AI in health”
08/12/2025

- Clinical research
From canine odorology to electronic noses: a new way to detect breast cancer
27/11/2025



- Turquoise September
Institut Curie mobilizes to prevent, understand and treat gynecological cancers
04/09/2025
- Golden September
At Institut Curie, life goes on during treatment for children and their families
01/09/2025