- Home >
- Institut Curie News >
- Macrophage populations in breast cancer
It is now well established that the immune system play crucial roles in breast cancer. When the immune system is inhibited by the tumor environment, tumors can grow freely. But when correctly activated, immune cells impede tumor development. The role of macrophages - immune cells subtype installed in tissues - in these processes has yet to be understood. Researcher in the field generally consider macrophage infiltration in tumors to be a bad sign. But the work of researchers from Institut Curie describe a more complex picture. They show that a macrophage sub-population associates with an active immune response directed against the tumor and with increase survival of patients.
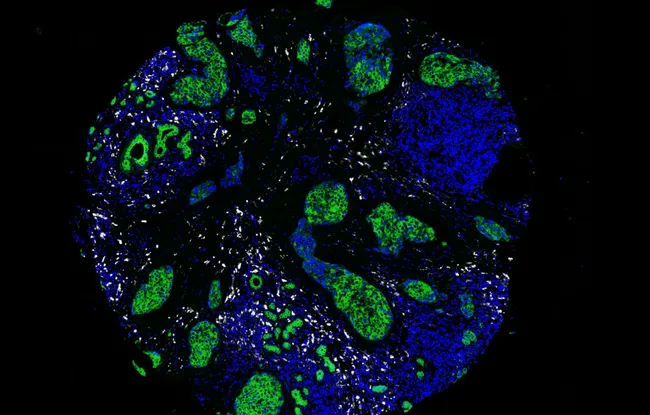
In a study published in Cell, Julie Helft, Rodrigo Nalio Ramos and Eliane Piaggio, from the Translational Immunotherapy team in the Immunity and Cancer unit (U932, Institut Curie, Inserm), in collaboration with the pathology and surgery departments at Institut Curie’s Hospital Group, and with other European laboratories, studied the macrophages present in the tumors of patients with luminal breast cancer, which is the most common form. Using a single-cell analysis, the biologists identified two macrophage populations. They explain. that “One of the macrophage population is located quite close to the cancerous cells, while the other one is more at the edge of the cancer”. Clinical and biological data from close to 80 patients[1] treated at Institut Curie show that the first associate with a poor prognosis while the second correlates with better chances of survival. So not all in infiltrated macrophages are the same; some are beneficial and others are not. “This contradicts the current paradigm! We were not expecting it,” explain the researchers.
To understand why some macrophages associate with anti-tumor immune responses, the researchers conducted in vitro studies on animal cells. “We demonstrated in vitro that the beneficial macrophages activate effector cells of the immune system.” The researchers also believe that these macrophages - cells residing in healthy tissue - are the first line of defense when the tumor begins to form. Then, if the cancer develops, they are diluted by the arrival of the second type of macrophages which appear to facilitate tumor growth by inhibiting immune responses.
It would be interesting to quantify and characterize the different macrophage sub-populations in order to evaluate the prognosis for patients and anticipate their responses to treatment,
explains Julie Helft, who led the project and is now team leader at Institut Cochin.
She is going to continue her collaboration with the team led by Eliane Piaggio at Institut Curie’s Research Center, and is already thinking about how to target these cells to develop treatments that improve patient immune response, in addition to current therapies.
Reference:
Tissue-resident FOLR2+ macrophages associate with CD8+ T cell infiltration in human breast cancer. Rodrigo Nalio Ramos, Yoann Missolo-Koussou, Yohan Gerber-Ferder, Pierre Guermonprez, Eliane Piaggio, Julie Helft. March 23, 2022 Cell Doi https://doi.org/10.1016/j.cell.2022.02.021
[1]All of these data are collected in accordance with current regulations, in particular the General Data Protection Regulation (GDPR) and the French law on personal data protection (Loi Informatique et Libertés).