- Home >
- Institut Curie News >
- Structure of Resting Cardiac Myosin Revealed
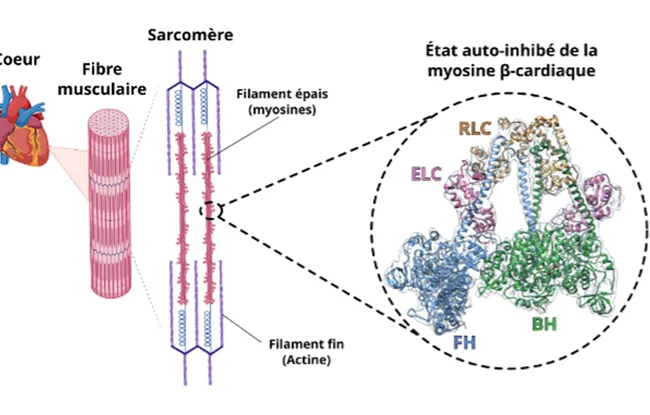
Cardiac muscle contraction is controled by the molecular motor myosin, which interacts with another protein, actin. To regulate the strength of its contractions, the heart adjusts the number of active motors as required. Myosin can adopt a “resting” state: the sequestered state, which is a specific structure in which two motors inhibit each other. But this state had never been observed up close... until now. Institut Curie's Structural Motility team has managed to obtain a high-resolution image.
To do so, researchers used cryogenic electron microscopy. The principle? A sample of protein is frozen very rapidly at a very low temperature and then passed through an electron beam. The image of each protein can then be reconstructed individually.
We selected a sample of 200,000 proteins in all orientations and then projected them to reconstruct the protein's 3D structure in its sequestered state
explains Dr Julien Robert-Paganin, CNRS research fellow in the Structural Motility team (CNRS UMR144 / Sorbonne University).
Better Understanding Cardiac Muscle
This achievement called for equipment from the European Synchrotron Radiation Facility in Grenoble.
Thanks to this study, our laboratory is now trained in this highly complex technique. As soon as the electron microscope acquired by Institut Curie in February is operational, we will be able to use it to continue our research on site.
adds Dr Anne Houdusse-Juillé, head of the Structural Motility team (CNRS UMR144 / Sorbonne Université).
The discovery of the structure of cardiac myosin in its sequestered state has already had far-reaching consequences. Firstly, it provides fundamental understanding of how heart muscle works.
Observing this sequestered state at high resolution shows that it is not very stable, unlike smooth muscle myosin. This makes sense: heart muscle must be able to control contractions far more quickly than muscles in the digestive system, for example
continues Julien Robert-Paganin.
Treatments to Stabilize the Sequestered State
The second consequence is directly medical: heart muscle contraction defects are the cause of familial hypertrophic cardiomyopathies (FHCM), which affect around one in 500 people.
We knew that mutations in cardiac myosin were responsible for this disease. But we didn't know if these mutations made the myosins more active or in fact prevented them from reaching or remaining in the sequestered state. Our study shows that the second hypothesis is the right one. Stabilizing this state is therefore a therapeutic objective, and by obtaining a high-resolution image of the protein's structure, this now seems more achievable
recalls Anne Houdusse-Juillé