Presentation

In breast cancer, transition of ductal carcinoma in situ (DCIS) to invasive ductal carcinoma (IDC) is associated with increased metastatic risk and drop in survival rate.
The invasive switch of breast cancer requires tumor cells to cross the basement membrane (BM), a specialized extracellular matrix (ECM) that separates epithelial cells from the stroma. Following BM transmigration, invading tumor cells migrate through three-dimensional (3D) type I collagen networks that can act as physical barriers against invasive migration of tumor cells. Invasive migration requires proteolytic remodeling of ECM barriers by cancer cells, which is executed by matrix-degrading proteases. Membrane-tethered 1 Matrix Metalloproteinase (MT1-MMP) is a key enzyme responsible for degradation of specific pericellular ECM substrates including fibrillar type I collagen and BM. We aim at understanding the molecular and cellular mechanisms underlying the invasive and metastatic programs of breast tumor cells.
Based on the Incentive and Cooperative Program “Breast cancer Invasion and Motility” launched by Institut Curie (coordinated by Drs A Vincent-Salomon & P. Chavrier) we analyzed the expression of MT1-MMP by immunohistochemistry in a TMA comprising ~500 breast tumors and found that MT1-MMP is up-regulated at the transition of DCIS to infiltrating breast lesions and is associated with higher metastatic risk. Studies performed in collaboration with pathologists and bioinformaticians from Institut Curie revealed the prognostic value of co-upregulation of MT1-MMP and several components of an exocytic machinery involved in MT1-MMP surface delivery in high-grade hormone receptor-negative breast tumors.
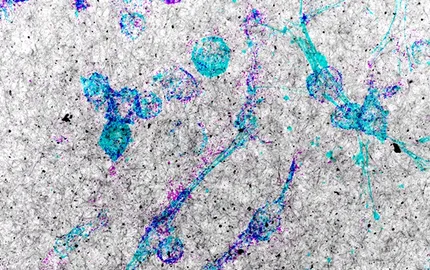
Earlier work established that MT1-MMP is recruited to actin-based matrix proteolytic structures called invadopodia. How mechanisms of actin assembly, signaling and MT1-MMP trafficking are integrated to produce the ECM degrading machine of invasive tumor cells is poorly understood and has been the focus of our studies during the past years. By modulating expression of regulators of MT1-MMP traffic combined with high-resolution cell imaging approaches we have generated a structural and functional map of invadopodia. Our recent data support a model whereby late endosomes serving as storage MT1-MMP compartments establish dynamic membrane tubular connections with the invadopodial plasma membrane, allowing exocytosis and accumulation of MT1-MMP to invadopodia.
We also found that epithelial polarity factors are diverted from their physiological functions in normal breast epithelial cells and contribute to the invasive capacity of breast tumor cells by controlling the trafficking and exocytosis of MT1-MMP. Polarity proteins involved in MT1-MMP traffic to invadopodia include the Rho GTPases Cdc42 and RhoA, the microtubule and actin crosslinker protein IQGAP1 and the oncogenic and polarity protein atypical protein kinase C iota. We investigated the function of microtubules during the polarized trafficking of MT1-MMP and identified an important role of tubulin post-translational modifications and microtubule-based motors in MT1-MMP function. Our most recent findings point to the unprecedented idea that invadopodia formation and matrix remodeling by MT1-MMP are adaptive responses triggered by mechanical confinement of tumor cells in the 3D collagen environment
In order to validate mechanisms based on studies with breast cancer-derived cell lines in vitro, we set-up an intraductal human-in-mouse orthotopic xenograft model that revealed that MT1-MMP is required for the BM transmigration and for the invasive switch of breast cancer.