- Home >
- Institut Curie News >
- The physical constraints of cellular environment regulate immunity
Dendritic cells (DCs) are a class of immune system cells whose function is to scour the body for dangers. Once they have completed their patrols, DCs transport the samples they have collected to the lymph nodes, where they are presented to lymphocytes in the form of protein fragments. If this process takes place in a “dangerous” context, such as an infection or cancer, it will activate lymphocytes, which in turn eliminate the infectious agent or tumor cell. On the contrary, if it takes place in the absence of danger, it will inactivate self-reactive lymphocytes, thus avoiding autoimmune reactions. This phenomenon is known as “self-tolerance maintenance”.
“In order to perform their functions, DCs must be able to move through all the different kinds of tissues that make up the body. Specialized sensing mechanisms are present in DCs so that they can adapt to the physical constraints of their environment. This adaptation requires the activation of certain cellular pathways. We wondered whether activation of the sensing pathway had an impact on DC function itself", explains Dr Zahraa Alraies, first author of this work and former postdoctoral researcher in Ana-Maria Lennon-Duménil's team.
Guides of cell migration
In their previous work, Matthieu Piel and Ana-Maria Lennon-Duménil's teams demonstrated that deformation of the cell, and more specifically the nucleus, induced a change in DC speed. This ground-breaking work established an initial link between the mechanical forces exerted on DCs and their surveillance and migration activity.
Researchers now unraveled the signaling pathway responsible for detecting physical environmental stress. Activation of this signaling pathway leads to two phenomena: on the one hand, an increase in DC migration speed and directionality, and on the other, an increase in the expression of a surface receptor called CCR7, which guides DCs to lymph nodes. Together, these two processes enable DC steady-state[1] migration.
"For decades, the scientific community has been trying to understand which biochemical molecules enable DC migration under homeostatic conditions. However, our work suggests that this migration responds to the physical constraints of the environment,” adds Zahraa Alraies.
Immune response, tolerance and the balance between the two
Homeostatic migration of DCs to ganglia is essential to maintain immune tolerance, limiting reactions against non-pathogenic elements. DC functions thus play a part in preserving this balance between immune tolerance and response, and therefore, more broadly, our immunity. The results published in this article show that the physical properties of our tissues play a part in this phenomenon, under conditions of homeostasis. But this could also be the case in certain diseases, such as cancer. Indeed, solid tumors are associated with tissue rigidification, and therefore, most probably, with increased deformation of circulating DCs, modifying their migration behavior, and thus the balance between tolerance and immune response.
"Understanding how these altered physical properties influence immune responses could, for example, help us understand why immune responses against cancers don't always work effectively. This very promising line of research could also extend to vaccinations, where the very injection of the substance produces a mechanical pressure in the tissues, or to autoimmune diseases, for example, which can develop as a result of a breakdown in the balance between immune response and tolerance. This opens up a huge number of possibilities for exploration,” concludes Zahraa Alraies.
|
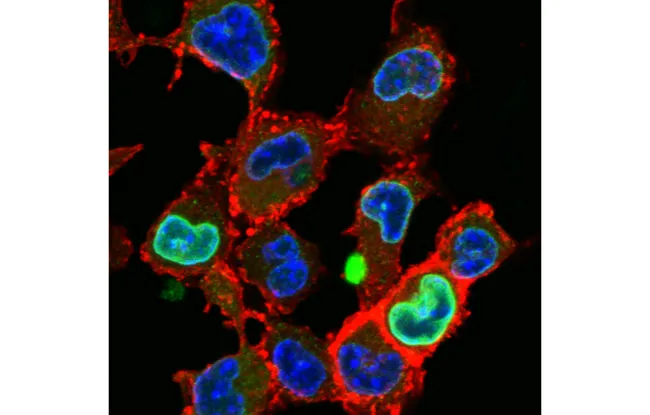
Image description: Immunostaining of CCR7 (in red) in constrained dendritic cells. These results were obtained with funding from the ERC Synergy for Project 101071470 - SHAPEINCELLFATE. References: Zahraa Alraies, Claudia A. Rivera, Maria-Graciela Delgado, Doriane Sanséau, Mathieu Maurin, Roberto Amadio, Giula Maria Piperno, Garett Dunsmore, Aline Yatim, Livia Lacerda Mariano, Anna Kniazeva, Vincent Calmettes, Pablo J. Sàez, Alice Williart, Henri Popard, Matthieu Gratia, Olivier Lamiable, Aurélie Moreau, Zoé Fusilier, Lou Crestey, Benoit Albaud, Patricia Legoix, Anne, S. Dejean, Anne-Louise Le Dorze, Hideki Nakano, Donald N Cook, Toby Lawrence, Nicolas Manel, Federica Benvenuti, Florent Ginhoux, Hélène D. Moreau, Guilherme P.F. Nader, Matthieu Piel and Ana-Maria Lennon-Duménil Cell Shape Sensing Licenses Dendritic Cells for Homeostatic Migration to Lymph Nodes. Nature Immunology (June 4, 2023). doi: 10.1038/s41590-024-01856-3 Link: https://www.nature.com/articles/s41590-024-01856-3
|
[1] Steady-state : when the body is not actively fighting against an infection or an inflammation