- Home >
- Institut Curie News >
- Immunotherapy, a new ally against cervical cancer?
Cervical cancer is the second cause of cancer death worldwide in women ages 20 to 39. When it is locally advanced, the standard treatment involves a combination of chemotherapy and radiotherapy, but patients remain exposed to a 40% risk of recurrence. “Our ambition is to find more effective treatment combinations”, announced Dr. Emanuela Romano, medical director of Cancer Immunotherapy Center at Institut Curie
Together with a team of researchers, oncologists, radiotherapists and surgeons, Dr. Manuel Rodrigues, medical oncologist and researcher in the DNA Repair and Uveal Melanoma (D.R.U.M) team (Inserm U830), and Dr. Emanuela Romano looked at the combination of this classic treatment with immunotherapy. Immunotherapy involves targeting the immune response, or more precisely, blocking it. This occurs naturally in the human body: checkpoints on tumor cells may limit the immune response. And tumor cells take advantage of this process to escape the immune system.
To block... the block
The researchers’ idea? To inhibit these checkpoints so that the tumor cannot escape the immune response. Dr. Romano and Dr. Rodrigues and their colleagues tested a combination of chemoradiotherapy with immunotherapy in 16 patients with locally advanced cervical cancer, using nivolumab, a checkpoint inhibitor. “The main goal was to check the tolerance and safety of the treatment”, explain the physicians. And this goal was achieved, since the 240 mg dose of nivolumab, administered every two weeks for the five weeks of chemotherapy, and then for the next six months, did not cause any serious side effects.
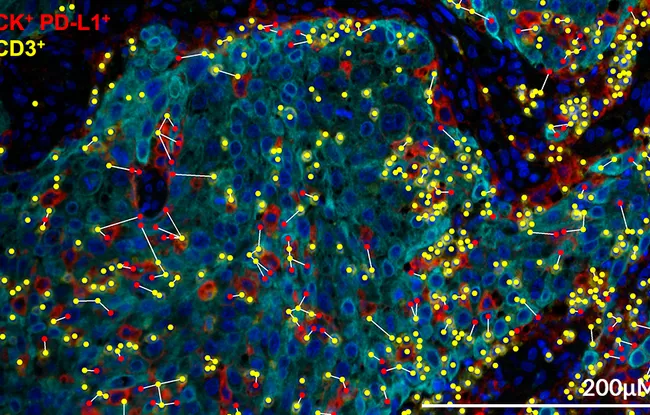
T lymphocytes: potential biomarkers
This research also provided more good news, in that it revealed biomarkers that can potentially predict the efficacy of this new treatment. Researchers found that the patients who responded best were those who had T-lymphocytes already present in the tumor from the outset, and co-stimulatory molecules. “It is the sign that their immune system has already fought cancer”, explains Dr. Giulia Vanoni, post-doctoral research in the Immune Responses to Cancer team (Inserm U932), who was particularly involved in the laboratory studies. “And nivolumab helps to bolster this response.” Conversely, patients that did not respond well had more regulating lymphocytes, a sign of systemic immunosuppression.
Selecting patients with the former profile would enable a clinical trial to assess this time the efficacy of this new combination. In this first study, efficacy is almost identical to that obtained with chemotherapy alone (a 2-year survival rate without progression of 75%), “but the sample is too small to be representative”, indicates Dr. Romano.
While awaiting this next step, physicians and researchers at Institut Curie continue to follow-up with their patients: the long-term survival rate without relapse will also be an indicator in favor of or against including nivolumab in the therapeutic toolbox.