- Home >
- Institut Curie News >
- Triple-negative breast cancer: new data in the regulation of the mTOR pathway
Among the treatments offered to patients with triple-negative breast cancers, certain molecules target a specific cellular pathway, which is hyperactivated in these tumors. A study conducted by Dr. Philippe Chavrier, research director at the CNRS and team leader (CNRS UMR144 / Sorbonne university) at Institut Curie, and published in Developmental Cell on December 26, 2024, show that the pharmacological blocking of this cellular pathway increases the degradation of the extracellular matrix, and could promote the invasive behavior of cells.
Triple-negative breast cancers, which account for 15% of all breast cancers (source INCa 2023), affect young women in particular, and they remain difficult to treat. The development of effective and innovative therapies against this cancer is essential to treat the patients suffering from it. Institut Curie, through its Institut hospitalo-universitaire (IHU) Institute of Women's Cancers, has made it one of its central research topics.
In a large fraction of cancers, a cellular pathway, named PI3K/AKT/mTOR, is hyperactivated. One of the lines of therapy proposed to patients involves inhibiting the activity of the mTOR protein complex, which in its turn blocks the cascade of reactions at the origin of tumor proliferation. The PI3K/AKT/mTOR pathway is frequently hyperactivated in triple-negative breast cancers, and ongoing clinical trials are targeting this pathway.
Using clinically relevant models of triple-negative breast cancers, the works published on the website of Developmental Cell and led by Dr. Philippe Chavrier, head of the Membrane and Cytoskeleton Dynamics team (CNRS UMR144 / Sorbonne University) at Institut Curie, have demonstrated that inhibition of mTOR could induce degradation of the extracellular matrix, in particular by forming invadopods. These specialized cellular structures of tumor cells allow them to invade tissues. Invadopods therefore facilitate tumor invasion and increase the risk of metastasis.
By tracing the chain of reactions of the PI3K/AKT/mTOR pathway, the authors also observed that the repression of mTOR stimulated the overexpression of the transcription factor TFEB, which is also involved in the formation of invadopods. "Studies in triple-negative breast cancer show that targeting mTOR reduces cell proliferation. Our work raises several questions and in particular, that of the risk of making tumors more invasive," continues Philippe Chavrier.
Moreover, previous results from the same team, published in Advanced Sciences in 2021 demonstrated that a nutrient deficiency in cells promoted the repression of mTOR and activated the degradation of the extracellular matrix. "Our results suggest that blocking mTOR could strengthen the abilities of tumor cells to move. It is therefore necessary to expand the research on this subject, in order to understand the real clinical implications of mTOR inhibitors in the treatment of triple-negative breast cancers," concludes Philippe Chavrier.
This research was made possible thanks to the exceptional donation of one million euros from Trond S. Paulsen for the fight against tumor invasion (InvaCell program in collaboration with the Radium Hospital and the University of Oslo), and thanks to the support of the Pink Ribbon Association and the Fondation ARC.

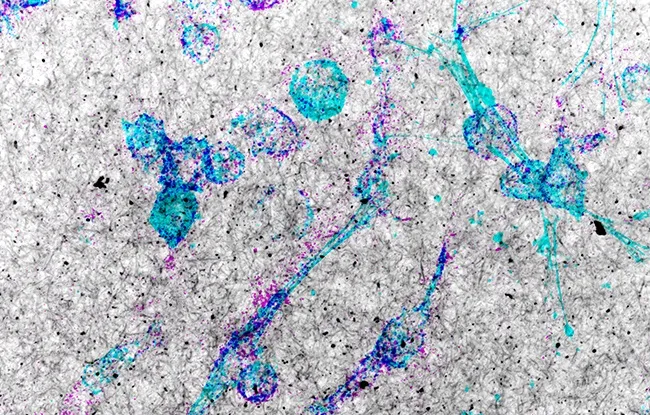
Figure: The image shows cancer cells from a tumor of a patient with triple-negative breast cancer. The cells (cyan) were treated with an mTOR pathway inhibitor (Torin-1) and incubated in a matrix composed of type I collagen fibers (black and white). The degradation of the collagen matrix, greatly increased by Torin-1 treatment, is revealed by staining visible in magenta.
Reference:
David Remy, Sandra Antoine-Bally, Sophie de Toqueville, Célia Jolly, AnneSophie Macé, Gabriel Champenois, Fariba Nemati, Isabel Brito, Virginie Raynal, Amulya Priya, Adèle Berlioz, Ahmed Dahmani, André Nicolas, Didier Meseure, Elisabetta Marangoni, and Philippe Chavrier. TFEB triggers a matrix degradation and invasion program in triple-negative breast cancer cells upon mTORC1 repression. Developmental Cell. December 26, 2024