Presentation

During development, the formation of functional tissues and organs entails both tissue shaping (morphogenesis) and cell proliferation; two processes driven by biochemical and mechanical regulation. In toto imaging of embryos highlighted the spatial and temporal coordination between these two processes: from the earliest stage of embryonic development, to later stages during which the collective dynamics of thousands of dividing and moving cells promote the emergence of tissues and organs. Conversely, deregulation of epithelial tissue morphogenesis or proliferation is associated with pathologies such as cancer, with approximately 90% of all human cancers having an epithelial origin.
Our general aim is to explore the mechanisms of morphogenesis and proliferation in epithelial tissues. We explore these mechanisms in the Drosophila dorsal thorax. This is monolayered epithelium that has been instrumental for the understanding of numerous fundamental processes ranging from gene patterning, tissue mechanics, division or cell polarization. During its development, this epithelium proliferates while undergoing several morphogenetic movements. As such, this tissue offers a unique system to decipher the genetic, mechanical, and self-organizing processes driving tissue shaping movements and the orchestration of more than 10.000 cell divisions. Using advanced methods in live-imaging, genetics, biophysics and bioinformatics combined with biophysical modeling, our work is organized around two main topics: i) exploring how cells divide in epithelial tissue and how epithelial polarity is maintained during division. ii) deciphering how gene expression, tissue planar polarity and mechanical forces collectively drive proliferation and morphogenesis.
Understanding cell division in epithelial tissues
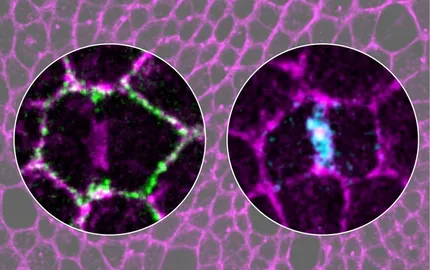
During cell proliferation, genome integrity entails the correct assembly of the mitotic spindle and cytokinesis completion. In epithelial tissues, cell proliferation is tightly controlled to maintain epithelial organization and tissue integrity. Accordingly, during epithelial cell division, the mitotic spindle is oriented in the plane of the tissue, and cytokinesis is coupled to epithelial repolarization to prevent cell delamination and polarity defects, which make cells prone to tumor formation. By studying spindle orientation and cytokinesis, we have uncovered that cell division is a multicellular process relying on the interactions between the dividing cell and its neighbors, with tricellular junctions being key mediators of their interactions and stress fibers. Using advanced live-imaging methods and optogenetic approaches, we are currently exploring the general mechanisms driving epithelial cell division and how cells communicate within a tissue at the level of tricellular junctions. More generally, our work aims at understanding how the cell and its first neighbors define a fundamental unit of a tissue.
Linking gene expression, cell dynamics and mechanical forces
To understand proliferation and morphogenesis at tissue-scale, we need to integrate gene expression regulation, cytoskeleton dynamics and mechanical forces. Towards reaching this important goal, we are developing advanced spatial transcriptomic methods to define gene expression patterns at tissue scale, allowing to infer gene candidates that are likely to regulate cytoskeleton organization and mechanical forces in specific tissue domains. In parallel, we are exploring how in turn the tissue mechanical stress and cell geometry modulates gene expression, proliferation, and apoptosis. In this context we have recently discovered a fundamental scaling property of cell mechanical response according to cell size. We are now exploring how cells measure their cell size to adapt their gene expression and mechanical response. These works integrating cell and tissue scale level regulations are powered by the implementation of advanced methodologies including single cell transcriptomics, optogenetics, quantitative biology and physical modelling. Collectively, we expect to provide an comprehensive view of the the biochemical and biomechanical principles sculpting tissues and organs during development.