- Home >
- Directory of the Institut Curie >
- EMMANUEL FARGE
Functions within Institut Curie
Team Leader
Presentation
Trained as a physicist (U-Paris7 Magistère, 1984-89), Emmanuel Farge switched to biophysics in the Physico-Chemistry Biology Institute Paris, in 1989 as a Ph.D. student. He worked on the soft matter elastic response of biological membranes to biochemical active trans-membrane translocation of phospholipids ("flippase"), leading to endocytic-like vesiculation on liposome mimetic model systems (PhD, 1993; Farge et al Bioph.J. 1992)
In 1994, he joined the Pasteur Institute in Paris (as assistant professor of U-Paris 7, 1993), where he showed the motor role of the "flippase" activity in endocytic vesiculation driving forces in living cells (Farge, Bioph. J. 1995; Farge et al., Am J.Physiol, 1999).
He then moved on to set-up a young investigator group at the Curie Institute in Paris in 1997 (Institut Universitaire de France, 99-04), in which was discovered the possibility to mechanically control gene expression and cell differentiation; this though mechanical inhibition of morphogen endocytosis (Rauch et al., Am. J. Physiol. 2002).
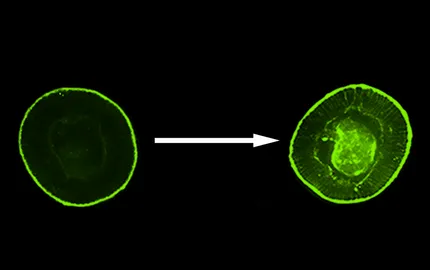
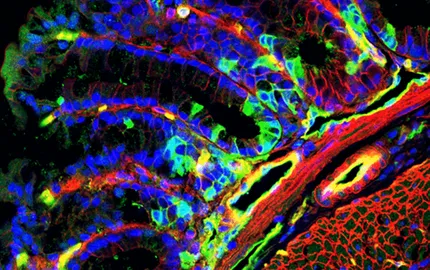
In parallel, the Drosophila embryo was introduced to study the feedback role of the mechanical strains developed by gastrulation morphogenetic movements into the regulation of developmental patterning genes expression. It was found the mechanical induction in Twist expression in the future anterior gut track cells, specifically strain compressed by the morphogenetic movement of convergent extension, and vital for functional anterior gut track development (Farge, Curr. Biol. 2003; Desprat et al Dev. Cell. 2008).
Currently, the Farge (Research Director Inserm, 2006) group studies biological molecular to mechanical multi-cellular phenotype interplay during embryonic morphogenesis and tumour development. This involves mechanotransduction driven cell collective effects leading to gastrulation (Pouille, Ahmadi et al. Science Sign. 2009; Mitrossilis et al. Nat. Com. 2017), with implications in the understanding of the evolutionary transition to mesoderm emergence in first bilateria (Brunet, Bouclet et al, Nat. Com. 2013), and in the molecular mechanisms underlying mechanical to biochemical signaling translation (Röper et al, eLife, 2018).
Health research implications consist in the finding of the reactivation of such ancestral embryonic mechanosensitive pathways in tumorigenic gene expression in the healthy tissues mechanically compressed by the growth pressure exerted by neighboring tumours (Fernandez-Sanchez, Barbier et al, Nature 2015).
Latest publications
Nature Biomedical Engineering - 20/12/2024
Nature Communications - 19/12/2024
Developmental Cell - 01/10/2023
Latest news
Contact EMMANUEL FARGE
Contact me by phone or by filling in the form below
0033156246760: 0033156246760